Recognizing the Importance of Patient Centricity
The term “patient centricity” is not new to those of us in the healthcare industry. Defined, it means putting patients at the core, by actively involving them across the multiple aspects of healthcare leading to enhanced care and outcomes. The patient centric ecosystem framework includes the needs of all relevant stakeholders, while the patient is at the center of the healthcare dynamics. I believe we should view patients as the center of the drug development and lifecycle management spectrum. Throughout my career in the pharmaceutical industry, approaching it through this lens has provided me with a unique perspective to ensure that patients are always the North Star.
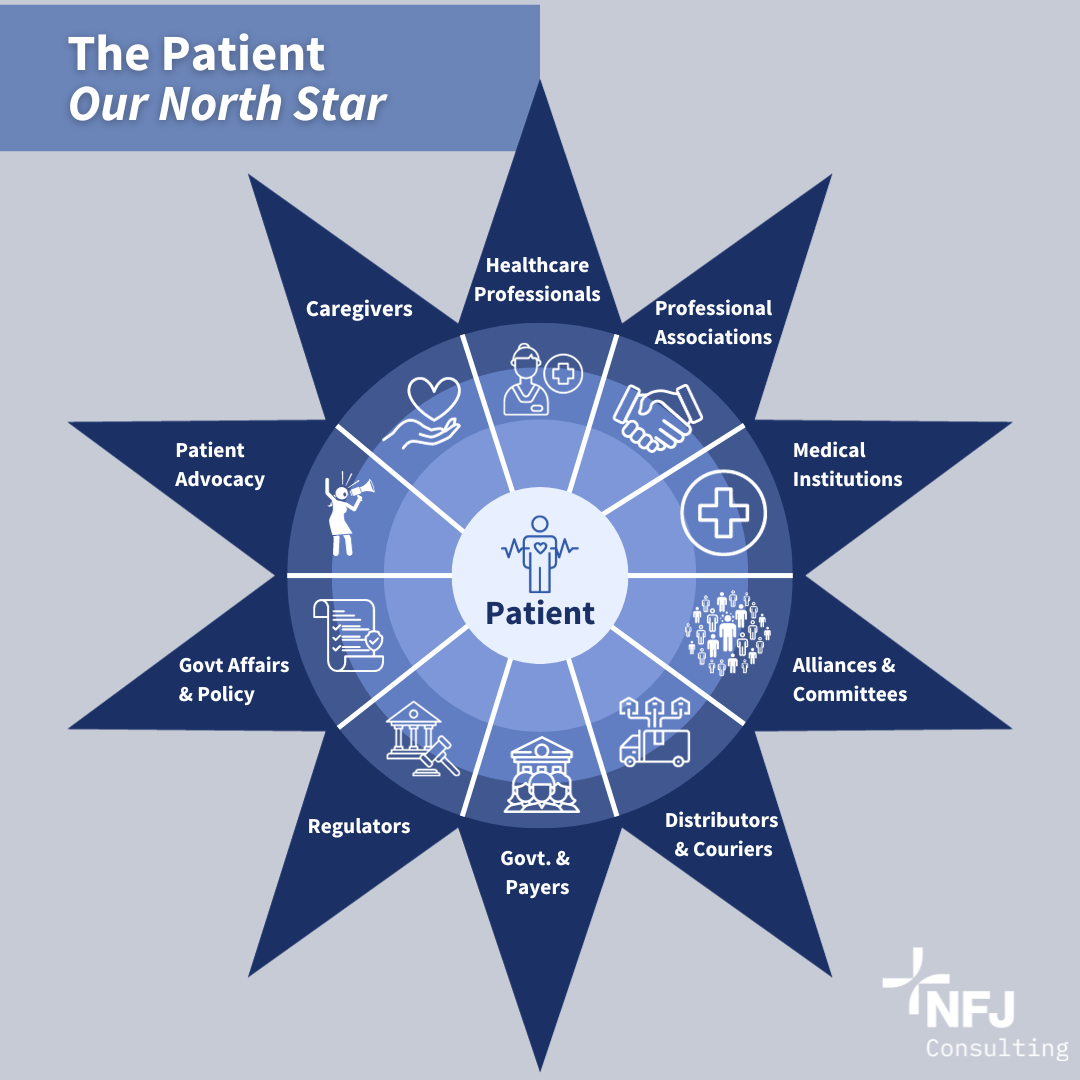
September 17, 2023, is World Patient Safety Day, and the importance of patient centricity is at the forefront as the World Health Organization (WHO) calls on all stakeholders to “take necessary action to ensure that patients are involved in policy formulation, are represented in governance structures, are engaged in co-designing safety strategies, and are active partners in their own care. World Patient Safety Day was established in 2019 as one of the WHO’s global public health days as a call for global action on patient safety. Four years later, the value of patient input and involvement is as important as ever. I have seen first-hand the evolution of patient centricity and how it involves actors in an arc that goes from the initial phases of drug discovery and development to the final steps of patient intake. I will describe what I have learned about what needs to happen when we put patients at the center of this continuum.
For a new drug to be successful, the unmet needs must be clearly defined and then subsequently met by the proposed solution of the new medication, as demonstrated through the development of a successful clinical program. Patient input is a crucial component throughout the process. A deep understanding of the natural history of a disease, the standard of care and the individual patient experience –uniquely comes from the ones affected with the condition and those who care for them. These insights are what drive a patient centric approach to the multifaceted journey of drug discovery, development and ultimately, availability. Moreover, an effective communication strategy to build the informed consent process and for the inclusion of trial subjects is required. To improve the odds of regulatory approval, that should be the final objective of development, it is important that the clinical trial design clearly contains patient information that is objectively associated to the intended clinical outcomes.
Insights Learned Along the Way
During my professional life one of my key areas of focus was the drug registration process. I had the opportunity of being part of teams that assembled drug registration packages for a great deal of countries across the world, including places like Brazil, China, Japan, Belgium, and Greece. While interacting and negotiating with registration authorities across those countries, and many others, the challenge remained the same: to demonstrate that a drug fulfils the requirements for efficacy, safety, and quality – for patients.
With each jurisdiction having its own specific requirements, it often became a lengthy, demanding, and challenging process from submission to eventual approval. But the one constant – across all the countries with which I engaged, was the patient. The quality of the data produced and submitted always had to be relevant to fulfill the patient need – data points proving a positive change for the patient journey. Demonstrating the impact of a drug on patients’ lives, enhances the likelihood it will be embraced by regulatory bodies. And having the ability to effectively communicate about this with regulatory authorities is essential.
To deliver safe and effective medications, there is no doubt that all the lengthy and expensive work done during drug development needs always to be done. And having the ability to share that data reflecting the patient experience, perspective, and input – through effective communication with regulatory authorities, is a critical component. Therefore, it is important to understand the different perspectives, cultural drivers, and requisites for additional data to present the patient perspective and demonstrated outcomes. I witnessed firsthand how doing this leads to success. Several examples standout for me, where drugs crucial to treating hematological malignances received approval based on their positive patient impact of dramatically changing the natural progression of cancer – even opening the door for a potential cure, while also reducing complications that patients had with previous existing treatments.
While the example I shared speaks specifically to the importance of the inclusion of patient data as part of the registration process for drug development, this is only one step in the entirety of its overall lifecycle. As noted by the #WHO, “evidence shows that when patients are treated as partners in their care, significant gains are made in safety, patient satisfaction and health outcomes.” Let us keep this in mind for World Patient Safety Day and beyond as we continue to advance drug development. And I look forward to doing that in future posts as I continue to explore the importance of patient centricity across the many facets of drug development and lifecycle management.